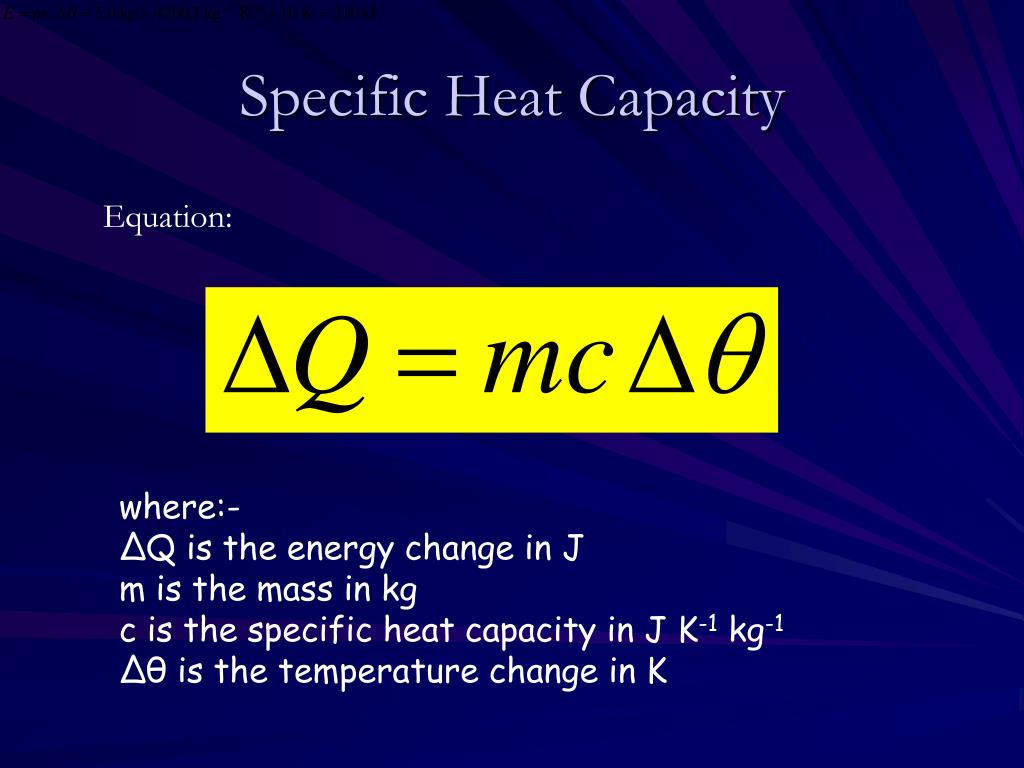
What Is Specific Heat Capacity Geography . specific heat capacity determines how much heat energy is needed to change the temperature of materials found in the. heat capacity is a measure of the heat required to raise the temperature of 1g of a substance by 1 celsius. specific heat capacity is the amount of heat energy required to raise the temperature of one unit mass of a substance by one. specific heat is defined by the amount of heat needed to raise the temperature of 1 gram of a substance 1. specific heat is defined as the amount of heat required to raise the temperature of a unit mass of a substance by one degree celsius. in thermodynamics, the specific heat capacity (symbol c) of a substance is the amount of heat that must be added to one unit of mass of the substance in.
from www.slideserve.com
specific heat is defined as the amount of heat required to raise the temperature of a unit mass of a substance by one degree celsius. specific heat capacity is the amount of heat energy required to raise the temperature of one unit mass of a substance by one. specific heat is defined by the amount of heat needed to raise the temperature of 1 gram of a substance 1. in thermodynamics, the specific heat capacity (symbol c) of a substance is the amount of heat that must be added to one unit of mass of the substance in. heat capacity is a measure of the heat required to raise the temperature of 1g of a substance by 1 celsius. specific heat capacity determines how much heat energy is needed to change the temperature of materials found in the.
PPT Specific Heat Capacity PowerPoint Presentation, free download
What Is Specific Heat Capacity Geography heat capacity is a measure of the heat required to raise the temperature of 1g of a substance by 1 celsius. specific heat capacity determines how much heat energy is needed to change the temperature of materials found in the. specific heat is defined by the amount of heat needed to raise the temperature of 1 gram of a substance 1. heat capacity is a measure of the heat required to raise the temperature of 1g of a substance by 1 celsius. specific heat is defined as the amount of heat required to raise the temperature of a unit mass of a substance by one degree celsius. specific heat capacity is the amount of heat energy required to raise the temperature of one unit mass of a substance by one. in thermodynamics, the specific heat capacity (symbol c) of a substance is the amount of heat that must be added to one unit of mass of the substance in.
From scienceinfo.com
Specific Heat Capacity Definition, Unit, Formula What Is Specific Heat Capacity Geography specific heat capacity determines how much heat energy is needed to change the temperature of materials found in the. specific heat is defined by the amount of heat needed to raise the temperature of 1 gram of a substance 1. in thermodynamics, the specific heat capacity (symbol c) of a substance is the amount of heat that. What Is Specific Heat Capacity Geography.
From www.youtube.com
What Is The Difference Between Specific Heat Capacity, Heat Capacity What Is Specific Heat Capacity Geography specific heat is defined by the amount of heat needed to raise the temperature of 1 gram of a substance 1. specific heat capacity determines how much heat energy is needed to change the temperature of materials found in the. specific heat is defined as the amount of heat required to raise the temperature of a unit. What Is Specific Heat Capacity Geography.
From www.downwindkennels.com
Specific Heat Capacity What Is Specific Heat Capacity Geography specific heat capacity determines how much heat energy is needed to change the temperature of materials found in the. specific heat capacity is the amount of heat energy required to raise the temperature of one unit mass of a substance by one. specific heat is defined as the amount of heat required to raise the temperature of. What Is Specific Heat Capacity Geography.
From www.youtube.com
Specific Heat and Heat Capacity YouTube What Is Specific Heat Capacity Geography in thermodynamics, the specific heat capacity (symbol c) of a substance is the amount of heat that must be added to one unit of mass of the substance in. specific heat capacity determines how much heat energy is needed to change the temperature of materials found in the. heat capacity is a measure of the heat required. What Is Specific Heat Capacity Geography.
From www.youtube.com
Specific Heat Capacity Short Example Solving for Mass YouTube What Is Specific Heat Capacity Geography specific heat capacity is the amount of heat energy required to raise the temperature of one unit mass of a substance by one. specific heat is defined by the amount of heat needed to raise the temperature of 1 gram of a substance 1. specific heat capacity determines how much heat energy is needed to change the. What Is Specific Heat Capacity Geography.
From askfilo.com
The differences between specific heat capacity and heat capacity are.. What Is Specific Heat Capacity Geography specific heat capacity is the amount of heat energy required to raise the temperature of one unit mass of a substance by one. heat capacity is a measure of the heat required to raise the temperature of 1g of a substance by 1 celsius. specific heat is defined as the amount of heat required to raise the. What Is Specific Heat Capacity Geography.
From studylib.net
1.3 Specific Heat Capacity What Is Specific Heat Capacity Geography specific heat is defined as the amount of heat required to raise the temperature of a unit mass of a substance by one degree celsius. heat capacity is a measure of the heat required to raise the temperature of 1g of a substance by 1 celsius. in thermodynamics, the specific heat capacity (symbol c) of a substance. What Is Specific Heat Capacity Geography.
From www.slideserve.com
PPT Specific Heat Capacity PowerPoint Presentation, free download What Is Specific Heat Capacity Geography specific heat capacity is the amount of heat energy required to raise the temperature of one unit mass of a substance by one. specific heat is defined by the amount of heat needed to raise the temperature of 1 gram of a substance 1. heat capacity is a measure of the heat required to raise the temperature. What Is Specific Heat Capacity Geography.
From slidetodoc.com
Specific Heat Capacity Objectives What is the difference What Is Specific Heat Capacity Geography in thermodynamics, the specific heat capacity (symbol c) of a substance is the amount of heat that must be added to one unit of mass of the substance in. specific heat is defined as the amount of heat required to raise the temperature of a unit mass of a substance by one degree celsius. heat capacity is. What Is Specific Heat Capacity Geography.
From www.youtube.com
Specific Heat Capacity Example Problem Physics YouTube What Is Specific Heat Capacity Geography in thermodynamics, the specific heat capacity (symbol c) of a substance is the amount of heat that must be added to one unit of mass of the substance in. specific heat is defined by the amount of heat needed to raise the temperature of 1 gram of a substance 1. specific heat capacity is the amount of. What Is Specific Heat Capacity Geography.
From www.tes.com
Specific Heat Capacity SAQs Teaching Resources What Is Specific Heat Capacity Geography specific heat is defined as the amount of heat required to raise the temperature of a unit mass of a substance by one degree celsius. in thermodynamics, the specific heat capacity (symbol c) of a substance is the amount of heat that must be added to one unit of mass of the substance in. heat capacity is. What Is Specific Heat Capacity Geography.
From studymind.co.uk
Heat Capacity Study Mind What Is Specific Heat Capacity Geography in thermodynamics, the specific heat capacity (symbol c) of a substance is the amount of heat that must be added to one unit of mass of the substance in. specific heat is defined by the amount of heat needed to raise the temperature of 1 gram of a substance 1. heat capacity is a measure of the. What Is Specific Heat Capacity Geography.
From gcsephysicsninja.com
40. Thermal capacity vs specific heat capacity What Is Specific Heat Capacity Geography specific heat is defined by the amount of heat needed to raise the temperature of 1 gram of a substance 1. specific heat is defined as the amount of heat required to raise the temperature of a unit mass of a substance by one degree celsius. specific heat capacity determines how much heat energy is needed to. What Is Specific Heat Capacity Geography.
From www.youtube.com
Specific heat capacity YouTube What Is Specific Heat Capacity Geography specific heat capacity is the amount of heat energy required to raise the temperature of one unit mass of a substance by one. specific heat capacity determines how much heat energy is needed to change the temperature of materials found in the. in thermodynamics, the specific heat capacity (symbol c) of a substance is the amount of. What Is Specific Heat Capacity Geography.
From www.slideserve.com
PPT Specific Heat Capacity PowerPoint Presentation, free download What Is Specific Heat Capacity Geography specific heat is defined by the amount of heat needed to raise the temperature of 1 gram of a substance 1. specific heat capacity determines how much heat energy is needed to change the temperature of materials found in the. in thermodynamics, the specific heat capacity (symbol c) of a substance is the amount of heat that. What Is Specific Heat Capacity Geography.
From study.com
Specific Heat Capacity Definition, Formula & Calculation Video What Is Specific Heat Capacity Geography specific heat capacity determines how much heat energy is needed to change the temperature of materials found in the. heat capacity is a measure of the heat required to raise the temperature of 1g of a substance by 1 celsius. in thermodynamics, the specific heat capacity (symbol c) of a substance is the amount of heat that. What Is Specific Heat Capacity Geography.
From physicscalculations.com
How to Calculate Specific Heat Capacity What Is Specific Heat Capacity Geography specific heat capacity determines how much heat energy is needed to change the temperature of materials found in the. heat capacity is a measure of the heat required to raise the temperature of 1g of a substance by 1 celsius. specific heat is defined as the amount of heat required to raise the temperature of a unit. What Is Specific Heat Capacity Geography.
From lessonlibcordwainer.z22.web.core.windows.net
High Specific Heat Capacity Meaning What Is Specific Heat Capacity Geography in thermodynamics, the specific heat capacity (symbol c) of a substance is the amount of heat that must be added to one unit of mass of the substance in. specific heat is defined by the amount of heat needed to raise the temperature of 1 gram of a substance 1. specific heat capacity determines how much heat. What Is Specific Heat Capacity Geography.